Hydrogen exponent (pH factor) Is a measure of the activity of hydrogen ions in a solution, quantitatively expressing its acidity. When the pH is not at optimal levels, plants begin to lose their ability to absorb some of the elements needed for healthy growth. All plants have a specific pH level that allows for maximum growing results. Most plants prefer a slightly acidic growth environment (between 5.5-6.5).
Hydrogen exponent in formulas
In very dilute solutions, the pH is equivalent to the concentration of hydrogen ions. Equal in magnitude and opposite in sign to the decimal logarithm of the activity of hydrogen ions, expressed in moles per liter:
pH = -lg[H+]
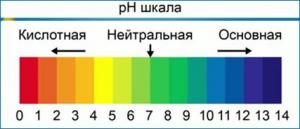
Under standard conditions, the pH value lies in the range from 0 to 14. In pure water, at neutral pH, the concentration of H+ is equal to the concentration of OH– and is 1 10-7 mole per liter. The maximum possible pH value is defined as the sum of pH and pOH and is equal to 14.
Contrary to popular belief, pH can vary not only in the range from 0 to 14, but can also go beyond these limits. For example, at a concentration of hydrogen ions [H+] = 10−15 mol / l, pH = 15, at a concentration of hydroxide ions [OH–] 10 mol / L pOH = −1.
It is important to understand! The pH scale is logarithmic, which means that each unit of change equals a tenfold change in the concentration of hydrogen ions. In other words, a pH 6 solution is ten times more acidic than a pH 7 solution, and a pH 5 solution will be ten times more acidic than a pH 6 solution and a hundred times more acidic than a pH 7 solution. means that when you are adjusting the pH of your nutrient solution and you need to change the pH by two points (for example, from 7.5 to 5.5), you should use ten times more pH corrector than if you were changing the pH by only one point (from 7.5 to 6.5 ).
PH Determination Methods
Several methods are widely used to determine the pH value of solutions. The pH can be roughly estimated using indicators, accurately measured with a pH meter, or determined analytically by acid-base titration.
Acid-base indicators
For a rough estimate of the concentration of hydrogen ions, acid-base indicators are widely used – organic dye substances, the color of which depends on the pH of the medium. The most famous indicators include litmus, phenolphthalein, methyl orange (methyl orange) and others. Indicators can exist in two differently colored forms – either acidic or basic. The color change of each indicator occurs in its acidity range, usually 1–2 units.
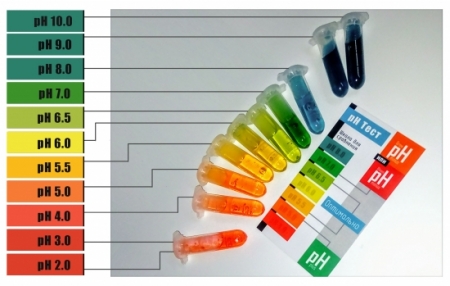
Universal indicator

The solutions of such mixtures – “universal indicators” are usually impregnated with strips of “indicator paper”, with the help of which it is possible to quickly (with an accuracy of pH units, or even tenths of pH) determine the acidity of the investigated aqueous solutions. For a more accurate determination, the color of the indicator paper obtained when applying a drop of solution is immediately compared with the reference color scale, the form of which is shown in the images.
Determination of pH by indicator method is difficult for turbid or colored solutions.
Considering the fact that the optimal pH values for nutrient solutions in hydroponics have a very narrow range (usually from 5.5 to 6.5), I also use other combinations of indicators. For example, our liquid pH test has an operating range and scale of 4.0 to 8.0, making it more accurate than a universal indicator paper.
pH meter

For a more detailed study of the topic, we recommend visiting the corresponding section of the forum: “pH meters”.
Analytical volumetric method
The analytical volumetric method – acid-base titration – also gives accurate results for determining the acidity of solutions. A solution of known concentration (titrant) is added dropwise to the test solution. When they are mixed, a chemical reaction occurs. The equivalence point – the moment when the titrant is exactly enough to completely complete the reaction – is fixed using an indicator. Further, knowing the concentration and volume of the added titrant solution, the acidity of the solution is calculated.
Effect of temperature on pH values

Adjusting the pH of the nutrient solution
Acidification of the nutrient solution
The nutrient solution usually needs to be acidified. The absorption of ions by plants causes a gradual alkalinization of the solution. Any solution having a pH of 7 or higher will most often need to be adjusted to the optimum pH. Various acids can be used to acidify the nutrient solution. Sulfuric or phosphoric acids are most commonly used. A better solution for hydroponic solutions are buffers such as pH minus Bloom and pH minus Grow. These products not only bring the pH values to the optimum, but also stabilize the values for a long period.
When adjusting the pH with both acids and alkalis, rubber gloves should be worn to avoid skin burns. An experienced chemist is adept at handling concentrated sulfuric acid, adding the acid drop by drop to the water. But for novice hydroponists, perhaps it is better to turn to an experienced chemist and ask him to prepare a 25% sulfuric acid solution. While the acid is being added, the solution is stirred and its pH is determined. Having learned the approximate amount of sulfuric acid, in the future it can be added from a graduated cylinder.
Sulfuric acid must be added in small portions so as not to acidify the solution too much, which will then have to be alkalinized again. In an inexperienced worker, acidification and alkalinization can go on indefinitely. In addition to wasting time and reagents, such regulation unbalances the nutrient solution due to the accumulation of ions unnecessary to plants.
Alkalinization of the nutrient solution
Too acidic solutions are made alkaline with caustic sodium (sodium hydroxide). As its name suggests, it is corrosive, so rubber gloves should be worn. It is recommended to purchase sodium hydroxide in pill form. In household chemical stores, sodium hydroxide can be purchased as a pipe cleaner, such as Mole. Dissolve one pellet in 0,5 L of water and gradually add the alkaline solution to the nutrient solution with constant stirring, frequently checking its pH. No mathematical calculations can calculate how much acid or alkali needs to be added in a given case.
If you want to grow several crops in the same pallet, you need to select them so that not only their optimal pH coincides, but also the needs for other growth factors. For example, yellow daffodils and chrysanthemums need a pH of 6,8, but different moisture conditions, so they cannot be grown on the same pallet. If you give daffodils as much moisture as chrysanthemums, the daffodil bulbs will rot. In experiments, rhubarb reached its maximum development at pH 6,5, but it could grow even at pH 3,5. Oats, which prefer a pH of about 6, give good yields at pH 4, if the nitrogen dose in the nutrient solution is greatly increased. Potatoes grow in a fairly wide pH range, but they thrive best at a pH of 5,5. Below this pH, high yields of tubers are also obtained, but they acquire a sour taste. To obtain maximum high quality yields, the pH of the nutrient solutions must be precisely adjusted.