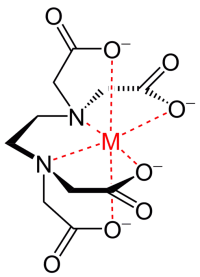
Chelates (chelating compounds) – chelate complex compounds formed by the interaction of metal ions with polydentate (that is, having several donor centers) ligands. Metal chelates are used as a source of trace elements in nutritional mixtures, due to the high absorption of chelate complexes in comparison with free metal ions.
The most popular are complexes of ethylenediaminetetraacetic acid – the abbreviation EDTA, less often EDTA (A-acetate acid is the synonymous name for acetic acid) (see Fig.).
Table 1
Metal dressing gowns used for the preparation of nutritional mixtures
Chelate Name Stability range, pH Ca-EDTA Ca-EDTA Ethylene diamine tetraacetate calcium Cu-EDTA Cu-EDTA Ethylenediamine tetraacetate copper 1,5-10 Fe-EDTA Fe-EDTA Ethylenediamine tetraacetate iron 1.5-6 Fe-EDDHA Fe-EDDHA Ethylenediamine dihydroxyphenyl acetate Fe-3.5 -EDDHSA 9-4 Fe-DTPA Fe-DTPA Ferrous diethylenetriamine pentaacetate 10-1.5 Fe-HEDTA 7-2.5 Fe-HBED 7-3.5 Mg-EDTA Mg-EDTA Magnesium ethylenediamine tetraacetate Mn-EDTA Mn-EDTA Manganese ethylenediamine tetraacetate 12-3 Zn-EDTA Zn-EDTA Zinc ethylenediaminetetraacetate 12-2 rus. English
The type of chelates depends on the pH of the solution. If the pH in the root zone is kept below 6.5, the Fe-DTPA chelate will provide sufficient stability. If the pH rises above 6.5, the use of Fe-EDDHA or Fe-HBED chelates is highly recommended.
Sources of
- Manual “Nutrient Solutions for greenhouse crops” available, 2016