Iron (chemical symbol Fe) is one of six micronutrients or trace minerals required for plant growth and reproduction. Of the many special properties of iron, its ability to readily undergo valence changes, or readily oxidize, is the essence of its biological importance. Most scientific publications and studies discuss iron in soil, where it is present as minerals (such as hematite), inorganic sediments (such as iron oxides), organic complexes (such as humates), and ions in the soil solution. Chemically, this occurs in two forms or oxidation states: Fe3+ and Fe2+… Ferrous iron is easily oxidized to ferric, which is practically insoluble in water. In normal, well-aerated agricultural soils, oxidation processes are actively occurring and, therefore, trivalent iron predominates. These phenomena are the main cause of the problem of iron deficiency in crops.
Like all plant nutrients, iron must be in an aqueous solution for the roots to absorb it. Any factor that decreases the activity or concentration of dissolved iron (Fe ions) will adversely affect absorption. This reaction is highly dependent on the pH level – the activity of soluble iron decreases 1000 times for each increase in pH by one.

Iron functions
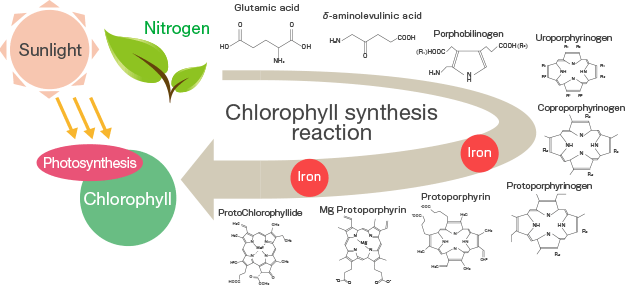
Plants require iron to produce chlorophyll and activate several enzymes, especially those involved in photosynthesis and respiration. It is also involved in protein synthesis and fruit color formation. Although the exact role of chlorophyll production is still unclear, a definite relationship between iron and chlorophyll content in plant leaves has been demonstrated. The interruption of chlorophyll production in iron-deficient plants is, of course, the cause of the universal visual symptom, chlorosis.
Iron is mainly absorbed by plants in the soil in the form of iron (Fe2+). However, since most agricultural soils contain iron in the form of iron (Fe3+), plants must somehow first dissolve Fe3+and then reduce it to Fe2 + so that it can pass through the plasma membrane of the hair root (plasmalemma). The exact mechanism that describes this process is still poorly understood. It appears to vary between plant species.
In most crops, iron uptake is an active process that requires energy. The root hairs of the plant secrete protons (ions H+) and exudates into the surrounding soil. Protons help dissolve Fe3+, lowering the pH, and promoting the chelation of Fe ions3+ phenolic exudates. On the root surface, iron chelate Fe3+ reduced to iron chelate Fe2+which easily releases Fe2+ for absorption by root hairs. As soon as he went to root, Fe2+ oxidized to Fe3+ and then chelated with citrate ions. The iron citrate chelate is then transported to actively growing areas of the plant. After translocation, iron tends to be fixed and cannot be re-transferred from organ to organ. For this reason, iron deficiency symptoms tend to only affect new growth.
Дефицит железа

Diagnosis and elimination of iron deficiency
Visual symptoms are common enough to accurately diagnose iron deficiency. If in doubt, you can spray with iron compounds – the reaction is usually very fast. The general effect of iron-deficient chlorosis is to reduce the photosynthetic activity required for growth and development. This, in turn, reduces crop yields and human economic use. Magnesium deficiency also shows chlorosis in the interveinal regions, but these symptoms begin on older leaves and the chlorosis is more yellow-orange in color. Manganese deficiency also shows chlorosis on younger leaves, but the veins remain green even with severe deficiency.
High levels of available molybdenum can reduce Fe uptake, causing iron molybdate to precipitate on the root surface. The most common cause of iron deficiency in plants is high pH – iron availability decreases when the pH is above 7. Iron deficiency can be caused by poor drainage of the substrate. Iron deficiency can also be caused by too much manganese.
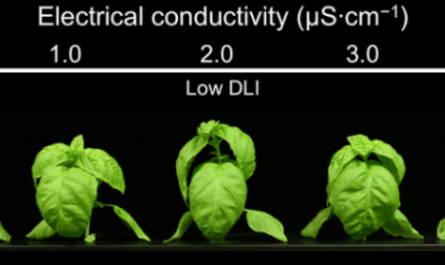
The optimum iron concentration for different plants varies. For example, for most grape crops grown, the nutrient solution should contain 2-3 ppm Fe (2-3 mg / L).
Excess iron
Iron buildup in cells can also be toxic. It can act catalytically to generate hydroxyl radicals that can damage lipids, proteins, and DNA. Because of the potential toxicity associated with high iron levels, cells store iron with an intracellular protein called ferritin, which releases iron in a controlled manner. This protein is produced by almost all living organisms, including algae, bacteria, higher plants and animals.
Diagnosis and elimination of excess iron
Iron toxicity mainly occurs where the pH drops enough to create an excess of available iron. As with some other nutrients, visible signs of iron toxicity are likely to be a sign of another nutrient deficiency. Iron accumulation can also occur with zinc deficiency. Excess iron can cause foliage to change color to a darker green.
Iron in nutrient solutions
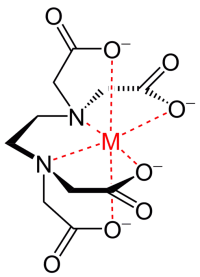
For hydroponics, ferrous sulfate (ferrous sulfate) or iron chelates are used as nutrients. Iron chelates are generally less prone to precipitation under alkaline conditions and are generally preferred for use. Read more in the article “Metal chelates”.
Sources of
- Practical Hydroponics & Greenhouses . September . 2016